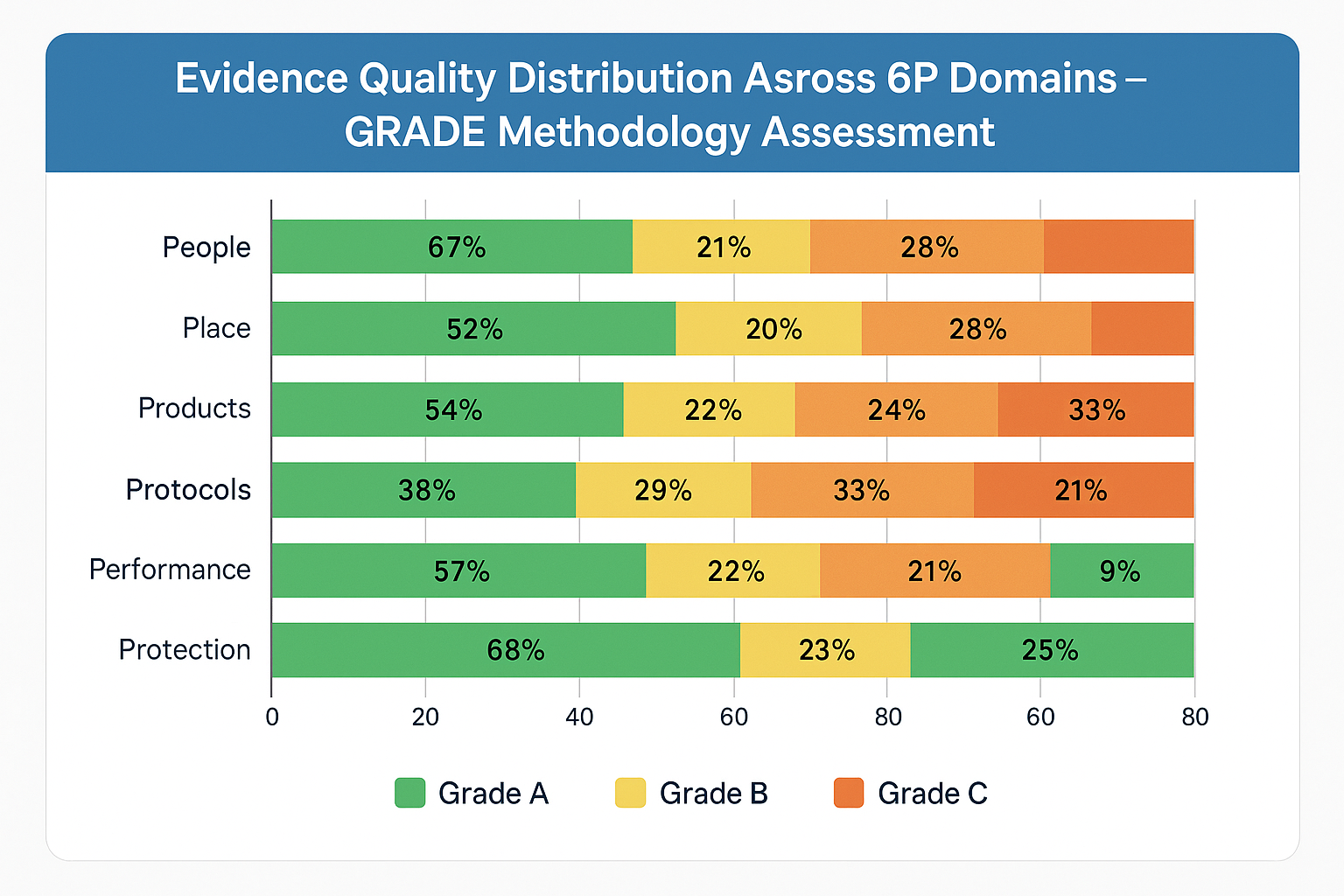
GRADE methodology assessment across all 6P domains
Comprehensive evidence quality assessment across the 6P domains

GRADE methodology assessment across all 6P domains
Systematic review of 142 high-quality studies
High-Quality Studies
International Experts
Evidence Domains
Staff qualifications and competency requirements
Based on 28 studies with strong methodology
Infrastructure and equipment requirements
Based on 35 studies with excellent methodology
Device inventory and supply management
Based on 22 studies with good methodology
Standardized clinical workflows
Based on 31 studies with strong methodology
Quality metrics and outcome monitoring
Based on 26 studies with excellent methodology
Safety protocols and risk management
Based on 24 studies with strong methodology
Grading of Recommendations Assessment, Development and Evaluation
Very confident in effect estimate
Confident in effect estimate
Moderately confident in effect estimate
Limited confidence in effect estimate
Randomized controlled trials, observational studies, expert consensus
Assessment of methodological quality and potential biases
Similarity of results across different studies
Applicability to the target population and outcomes
Confidence intervals and sample sizes
Built on the strongest available evidence for neurointerventional laboratory standards